Genome-level Analyses
My lab has a long-standing interest in understanding regulation of gene expression in the context of cognitive function. In earlier work, we identified the chromatin-modifier histone deacetylase 2 (HDAC2) as a master regulator of synaptic gene expression and showed that HDAC inhibitors enhance and restore learning and memory, demonstrating their therapeutic potential [1,2].
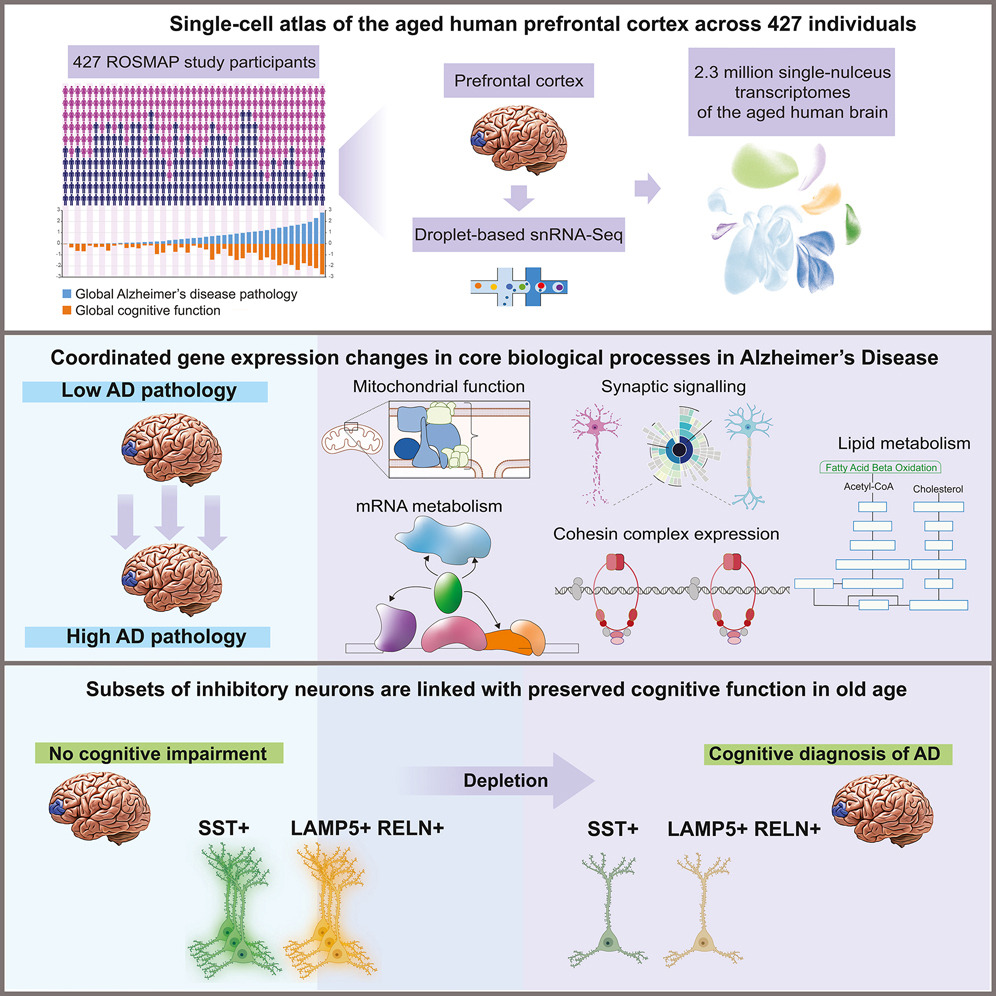
Some years ago, we turned to single-nucleus RNA sequencing (snRNAseq) of postmortem brain tissue to assemble a comprehensive atlas of cell-type specific gene expression changes associated with Alzheimer’s disease. Samples from both Alzheimer’s disease patients and age- and sex-matched cognitively normal controls were obtained from the longitudinal ROSMAP studies [3], which also collect rich metadata on participants. We performed snRNAseq on the postmortem prefrontal cortex from 392 individuals [4]. For 48 individuals, we sequenced five additional brain regions known to be affected in early (entorhinal cortex), intermediate (hippocampus and anterior thalamus), or late disease stages (angular gyrus, midtemporal cortex, and prefrontal cortex) [5]. In addition, we also performed single-nucleus ATAC-seq on postmortem prefrontal cortex samples from 92 individuals [6]. By performing clustering analysis of the RNAseq data and assigning clusters to brain cell types based on known cell-type specific marker genes, we were able to identify many distinct subtypes of inhibitory and excitatory neurons and delineate different microglial cell states [7]. The resulting genome-level gene expression atlas has recently been published, providing a comprehensive resource for the medical, scientific, and pharmaceutical communities.
Identifying determinants of vulnerability and resilience to Alzheimer’s disease
Based on our comprehensive snRNAseq data, we were able to identify several neuronal subtypes that were depleted in samples with Alzheimer’s disease, indicating increased vulnerability. These vulnerable neuronal subclasses included three subtypes of somatostatin (SST) inhibitory neurons as well as Reelin-expressing subtypes of excitatory neurons in the entorhinal cortex [4,5]. Notably, a gain-of-function variant in Reelin has previously been associated with resilience to familial Alzheimer’s disease [8].
The strongest association of gene expression with cognitive resilience to Alzheimer’s pathology was detected in astrocytes [5]. Remarkably, several of the resilience-associated genes coded for proteins involved in regulating intracellular choline availability. This finding strongly supports our independent results from in vitro studies that indicate the potential of choline supplementation as a therapeutic strategy for AD.
Role of DNA damage in Alzheimer’s disease
Double-strand breaks (DSBs) resolve topological constraints on the DNA and are necessary for the rapid expression of early-response genes in neurons [9]. However, while such physiological DSBs are typically repaired quickly, DSB accumulation is an early pathological hallmark of neurodegeneration. Based on snRNAseq on postmortem samples from human AD patients and the CK-p25 mouse model of neurodegeneration, we found that DSB accumulation leads to activation of antiviral inflammatory pathways and cellular senescence in neurons [10]. Neurons with senescence gene signatures displayed increased occurrence of gene-fusions that disproportionately affected DSB hotspots such as highly expressed or long genes [11]. snATACseq data from human postmortem samples further indicated a weakening of epigenomic cell identity – known as epigenomic erosion – and a break-down of epigenomic delineations between active and inactive genomic regions [6]. The predicted disruption of the normal 3D genome organization was validated using the Hi-C technique for detecting long-range chromosomal interactions [11]. Together, these finding strongly suggest a role for DNA damage accumulation and subsequent breakdown of chromosomal organization in Alzheimer’s pathogenesis.
Human microglial state dynamics in Alzheimer’s disease progression
Microglia are the resident immune cells of the brain. When activated, they secrete pro-inflammatory cytokines and become phagocytic. Since this normally protective response becomes damaging upon chronic activation in Alzheimer’s disease, chronically activated “disease associated microglia” (DAM) represent an important therapeutic target. While microglia are often poorly represented in snRNAseq data, our large dataset allowed us to identify 12 different microglial states including homeostatic, neuronal surveillance, lipid processing, phagocytic, stress related, glycolytic, and antiviral states as well as three different inflammatory states [7]. Surprisingly, the lipid processing state showed the most significant correlation with Alzheimer’s pathology and cognitive decline, and only one of the inflammatory states was significantly increased in Alzheimer’s samples. We also identified a series of transcription factors whose expression promoted the homeostatic state or whose inhibition weakened the inflammatory state, providing a roadmap for the development of therapies aimed at curbing neuroinflammation with disease-stage specificity.
References
- Guan, Ji-Song, Stephen J. Haggarty, Emanuela Giacometti, Jan-Hermen Dannenberg, Nadine Joseph, Jun Gao, Thomas J. F. Nieland, et al. 2009. “HDAC2 Negatively Regulates Memory Formation and Synaptic Plasticity.” Nature 459 (7243): 55–60.
- Gräff, Johannes, Damien Rei, Ji-Song Guan, Wen-Yuan Wang, Jinsoo Seo, Krista M. Hennig, Thomas J. F. Nieland, et al. 2012. “An Epigenetic Blockade of Cognitive Functions in the Neurodegenerating Brain.” Nature 483 (7388): 222–26.
- Bennett, David A., Aron S. Buchman, Patricia A. Boyle, Lisa L. Barnes, Robert S. Wilson, and Julie A. Schneider. 2018. “Religious Orders Study and Rush Memory and Aging Project.” Journal of Alzheimer’s Disease: JAD 64 (s1): S161–89.
- Mathys, Hansruedi, Zhuyu Peng, Carles A. Boix, Matheus B. Victor, Noelle Leary, Sudhagar Babu, Ghada Abdelhady, et al. 2023. “Single-Cell Atlas Reveals Correlates of High Cognitive Function, Dementia, and Resilience to Alzheimer’s Disease Pathology.” Cell 186 (20): 4365-4385.e27.
- Mathys, Hansruedi, Carles A. Boix, Leyla Anne Akay, Ziting Xia, Jose Davila-Velderrain, Ayesha P. Ng, Xueqiao Jiang, et al. 2024. “Single-Cell Multiregion Dissection of Alzheimer’s Disease.” Nature, July. https://doi-org.ezproxy.canberra.edu.au/10.1038/s41586-024-07606-7.
- Xiong, Xushen, Benjamin T. James, Carles A. Boix, Yongjin P. Park, Kyriaki Galani, Matheus B. Victor, Na Sun, et al. 2023. “Epigenomic Dissection of Alzheimer’s Disease Pinpoints Causal Variants and Reveals Epigenome Erosion.” Cell 186 (20): 4422-4437.e21.
- Sun, Na, Matheus B. Victor, Yongjin P. Park, Xushen Xiong, Aine Ni Scannail, Noelle Leary, Shaniah Prosper, et al. 2023. “Human Microglial State Dynamics in Alzheimer’s Disease Progression.” Cell 186 (20): 4386-4403.e29.
- Lopera, Francisco, Claudia Marino, Anita S. Chandrahas, Michael O’Hare, Nelson David Villalba-Moreno, David Aguillon, Ana Baena, et al. 2023. “Resilience to Autosomal Dominant Alzheimer’s Disease in a Reelin-COLBOS Heterozygous Man.” Nature Medicine 29 (5): 1243–52.
- Madabhushi, Ram, Fan Gao, Andreas R. Pfenning, Ling Pan, Satoko Yamakawa, Jinsoo Seo, Richard Rueda, et al. 2015. “Activity-Induced DNA Breaks Govern the Expression of Neuronal Early-Response Genes.” Cell 161 (7): 1592–1605.
- Welch, Gwyneth M., Carles A. Boix, Eloi Schmauch, Jose Davila-Velderrain, Matheus B. Victor, Vishnu Dileep, P. Lorenzo Bozzelli, et al. 2022. “Neurons Burdened by DNA Double-Strand Breaks Incite Microglia Activation through Antiviral-like Signaling in Neurodegeneration.” Science Advances 8 (39): eabo4662.
- Dileep, Vishnu, Carles A. Boix, Hansruedi Mathys, Asaf Marco, Gwyneth M. Welch, Hiruy S. Meharena, Anjanet Loon, et al. 2023. “Neuronal DNA Double-Strand Breaks Lead to Genome Structural Variations and 3D Genome Disruption in Neurodegeneration.” Cell 186 (20): 4404-4421.e20.
Video: Non-linear dimensionality reduction reveals global gene expression profile relationships across 73’909 individual cells isolated from the human brain. Legend below.