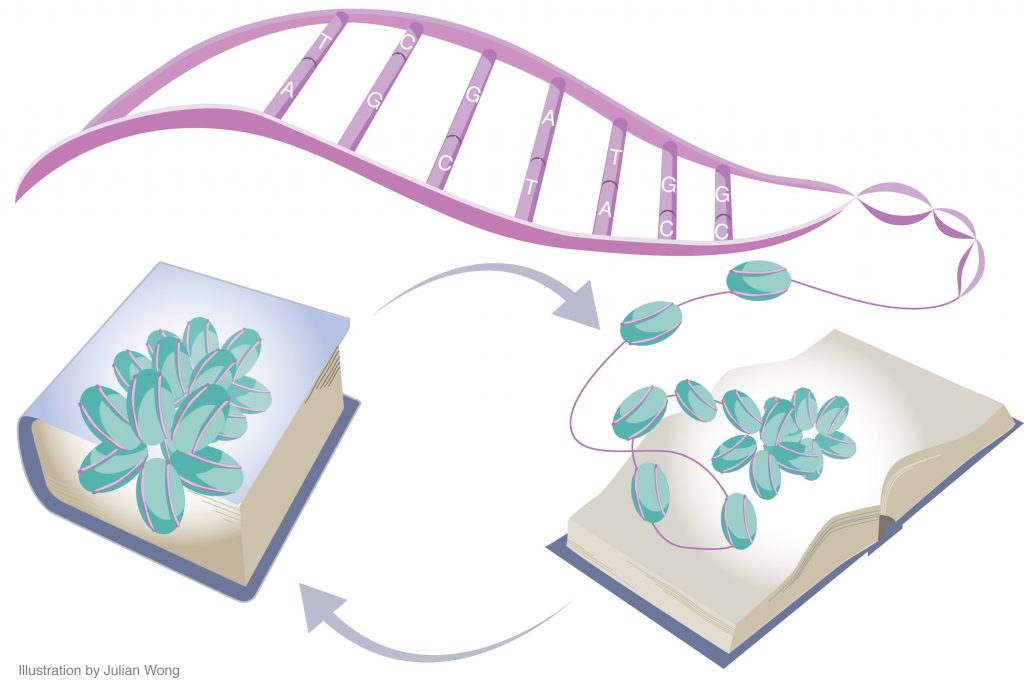
Following the completion of the Human Genome Project, much of biology’s focus has been shifted from the raw sequence of genes to their regulation over time and in response to environmental stimuli. Like books on a shelf, genes do not exert effects by their mere presence; rather, the pages of the book (i.e., the chromatin) need to be opened so that the words (i.e., the genes) can be read and interpreted correctly. The epigenetic regulation of gene expression refers precisely to this process.
In 1984, Francis Crick (1916-2004) proposed that memory might be coded in alterations to particular stretches of chromosomal DNA. Although the response to this idea was relatively modest at the time, the past decade has shown that chromatin, the carrier of chromosomal DNA, undergoes dynamic modifications and conformational changes during memory formation. One of these modifications is histone acetylation, the addition of an acetyl group to histone proteins. Histone acetylation is regulated by the opposing activities of histone acetyltransferases and histone deacetylases (HDACs) and generally closely correlates with active gene expression. Rodents display a transient increase in histone acetylation after exposure to various learning paradigms, and synaptic plasticity and memory formation are facilitated after treatment with small molecule inhibitors of HDACs.